Blog | Reading Time 6 minutes
Turnout to grass & live yeast benefits: What happens inside the rumen?
Interview with rumen microbiota expert, Frédérique Chaucheyras-Durand, Research Manager at Lallemand Animal Nutrition.
How does moving from a winter diet to a grass diet affect the rumen microbiota?
Any change in dietary conditions affects the rumen microbiota composition and activity. Indeed, the rumen microbiota is one that enables the ruminant animal to digest most of the diet components, in particular forages. At turnout to grass, animals are shifted from the winter diet (composed of starch concentrate and corn or grass silage most of the time) to lush grass with highly fermentable sugars and readily digestible cellulosic material. This increases the risk of Sub Acute Ruminal Acidosis (SARA). Lush grass is also high in soluble nitrogen.
Thus, rumen microbiota may be quite unstable with increased challenges for rumen health during the first weeks of the grazing period. The producer will probably spot this as loose manure, a visible indicator of SARA. Only a few studies have looked at microbial shifts that occur at grazing. Using the most recent DNA sequencing methods, we have a better understanding of what happens to rumen microbiota during the transition to a pasture diet, in particular:
- As young grass is rich in soluble sugars, there is a strong increase in lactate-producing bacteria, such as Streptococcus (Belanche et al., 2019), which puts the animal at risk for rumen acidosis and bloat.
- Cellulose and hemicellulose-degrading bacteria families — for example, Lachnospiraceae, of which Butyrivibrio fibrisolvens is an important member, and Ruminococcaceae —become abundant components of the microbiota, as well as Orpinomyces, which is an anaerobic rumen fungus (Guo et al., 2020).
- It also has been shown that proteolytic microbes, such as Prevotella and ciliate protozoa, increase response to the high levels of soluble nitrogen (Belanche et al., 2019), which may lead to an excess of ammonia production.
- The methanogenic Archaea Methanobrevibacter ruminantium is more abundant (Guo et al., 2020), which indicates that, as more cellulose is degraded, more methane may be produced. Thus, it is important to maximize feed efficiency to minimize the environmental impact of the grazing period.
Finally, it appears that an increase in bacterial diversity is observed when ruminants shift to a grazing diet, which may be linked with the need for a more complex microbial consortium to ensure good rumen function at grazing. This indicates again that this period may be really unstable.
In summary, the ruminant encounters deep changes in its rumen microbial population and digestive function at the start of the grazing period. Once adapted, it is important to maintain the fibrolytic microbial communities at active, high levels to ensure optimal fiber digestion and high feed efficiency.
You have conducted research both in vitro and in vivo with Saccharomyces cerevisiae CNCM I-1077 live yeast. The effects of this live yeast on fiber degradation improvement are now fully established with many forage types. How strong is this effect on a grass diet?
We conducted several experiments using in vitro models and in vivo, which led us to understand the impact of S. cerevisiae I-1077, in particular on fiber degradation. We have been able to quantify fiber colonization by bacteria and fungi on different forage types and showed that the live yeast enhances this process, which leads to a global increase in NDF degradation (NDFd) (Chaucheyras-Durand et al., 2016).
Fungi and bacteria can cooperate in the plant cell wall breakdown process. Fungi exert both a mechanical effect on the plant tissue, thanks to their filamentous network, and an enzymatic effect. Bacteria, once adhered to fiber particles, produce a wide set of carbohydrate-active enzymes (called CAZymes) that will further degrade these polysaccharides. Protozoa can also be quite active in a forage diet. We have shown — through a metatranscriptomic analysis of rumen samples from a cow fed a mixed diet composed mainly with grass silage — that genes encoding for protozoal CAZymes were highly expressed on this diet (Comtet-Marre et al., 2017). Lallemand has been involved in many cow trials where NDFd has been measured for a wide diversity of forages, from silages, hays, straws, and pasture.
Overall, a significant improvement in NDFd has been demonstrated with differences in the level of improvement according to the type of forage. For instance, NDFd was improved by 2.6 to 7.9 units in a range of pasture, grass hay, or grass silages samples (figure 1; Lallemand Animal Nutrition internal data). These differences may be explained by the action that the live yeast will have on rumen microbiota and its environment.
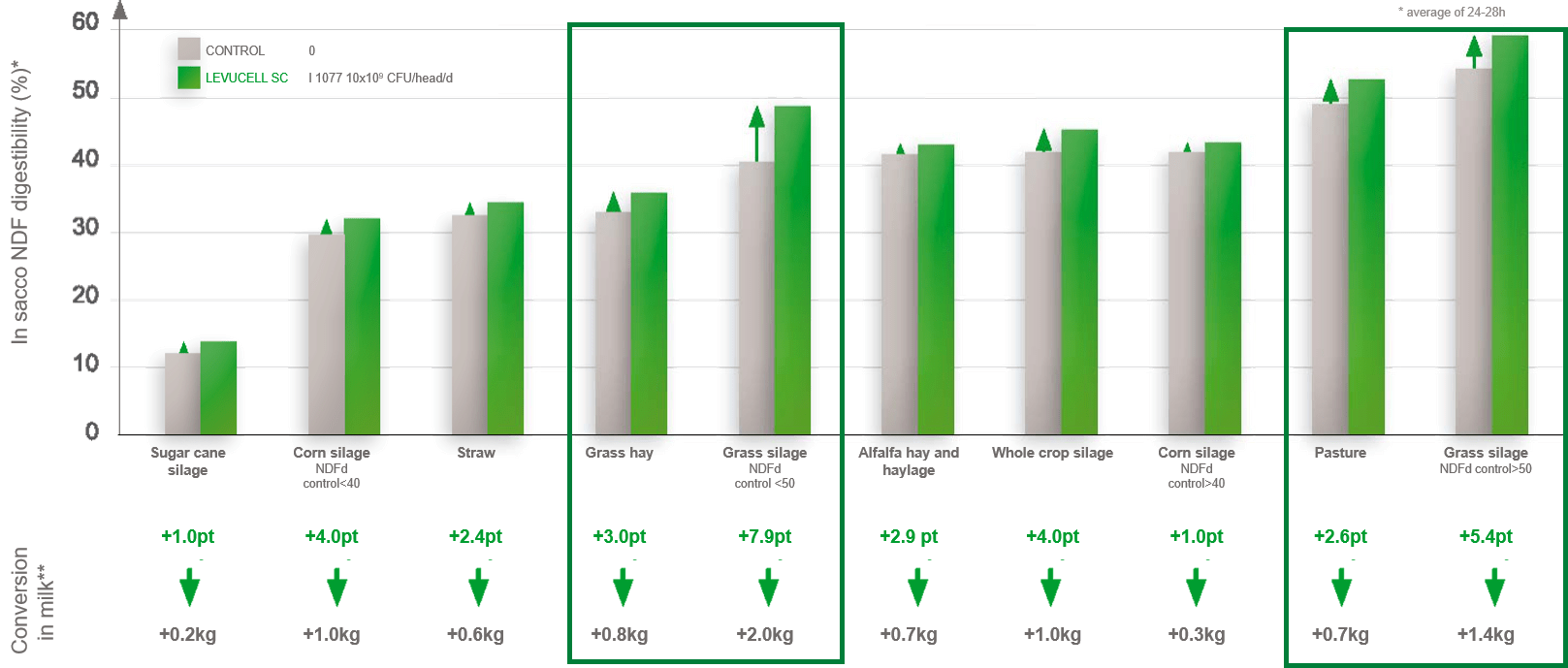
Figure 1: Effect of live yeast on NDF degradability for various forages (from Ding et al., 2014, Sousa et al., 2018, Guedes et al.,2010).
For this effect to be significant at the turnout to grass, when should we start feeding the live yeast?
It has already been shown that live yeast S. cerevisiae I-1077 takes less than one week to resolve sub-acidosis troubles caused by rapidly fermentable sugars (Bach et al., 2007). As mentioned at the beginning of this interview, any transition can be challenging for the rumen microbiome. To prevent any disturbance, it would be relevant to take a preventive approach by preparing the rumen microbiota a few weeks beforehand, and then feed the live yeast S. cerevisiae I-1077 during the full grazing period. This will help support SARA prevention at the turnout to grass. In the long run, this could help sustain improvement of NDF degradation to release more energy from the grass and contribute to better feed efficiency.
Bach, A., Iglesias, C., & Devant, M. 2007. Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Animal Feed Science and Technology, 136(1-2), 146-153.
Belanche, A., Kingston-Smith, A. H., Griffith, G. W., & Newbold, C. J. 2019. A multi-kingdom study reveals the plasticity of the rumen microbiota in response to a shift from non-grazing to grazing diets in sheep. Frontiers in microbiology, 10, 122.
Chaucheyras‐Durand, F., Ameilbonne, A., Bichat, A., Mosoni, P., Ossa, F., & Forano, E. 2016. Live yeasts enhance fibre degradation in the cow rumen through an increase in plant substrate colonization by fibrolytic bacteria and fungi. Journal of Applied Microbiology, 120(3), 560-570.
Comtet-Marre S, Parisot N, Lepercq P, Chaucheyras-Durand F, Mosoni P, Peyretaillade E, Bayat AR, Shingfield KJ, Peyret P and Forano E, 2017. Metatranscriptomics reveals the active bacterial and eukaryotic fibrolytic communities in the rumen of dairy cow fed a mixed diet. Frontiers in Microbiology, 8: 67
Ding G. et al, China Agricultural University, China, 2014 J. Anim. Sci. and Biotech. 5 :24,
Guedes et al., CECAV Portugal, 2010. Proceeding from Wageningen Symposium, Netherlands pp 25-30,
Guo, J., Li, P., Liu, S., Miao, B., Zeng, B., Jiang, Y., … & Zhang, H. 2020. Characterization of the rumen microbiota and volatile fatty acid profiles of weaned goat kids under shrub-grassland grazing and indoor feeding. Animals, 10(2), 176.
Sousa D.O., University of São Paulo, Brazil, 2018, Anim Feed Sci Technol 236:149-158
Published Mar 11, 2021 | Updated Dec 5, 2023
Related articles
Need specific information?
Talk to an expert