Blog | Reading Time 8 minutes
Inside a bacteria plant: the art and science of producing quality bacteria
Bacteria were among the first life forms to appear on Earth and they exist under a plethora of forms adapted to any environment, or inside our bodies. Certain strains have been carefully selected for specific industrial or food applications and are produced in fermentation facilities.
For the past decades, the use of live bacteria for animal production has gained momentum. They are the active ingredients of probiotics, and silage inoculants but also novel animal environment solutions such as positive biofilm solutions and bioremediation products used in aquaculture.
From a single vial of a master cell bank to a packaged bacteria product, here is the journey of bacteria cells inside one of Lallemand Animal Nutrition’s dedicated production plants.
Controlling every step
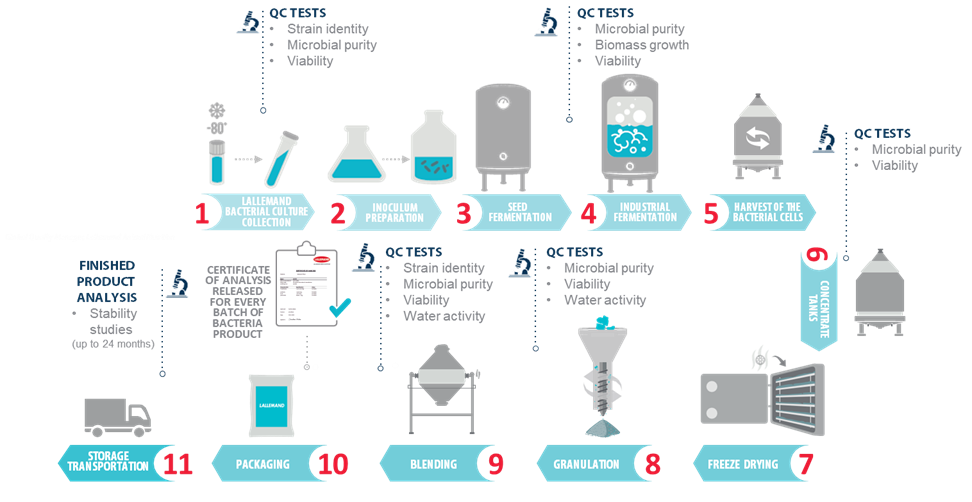
Several hundred tons of products containing a hundred billion live bacteria per gram are produced each year in a bacteria plant. However, it always starts with a few hundred microliters from a cell bank vial of a specific strain. This tiny number of live bacteria undergoes sequential multiplications in strictly controlled and sterile conditions, starting in a lab flask, all the way up to industrial fermenters (Figure 1).
With this in mind, it is easy to understand the importance of stringent quality control (QC) tests along the process: any contaminant or changes in growth conditions that will affect bacteria behavior or physiology could lead to undesired microorganisms multiplication and affect the end product purity, activity, and efficacy.
Throughout the whole production process, from the working cell bank that is used to start production to the logistics platform, at least 16 quality tests are performed ensuring, purity, viability, and identity of the bacteria strain in the final product, as well as batch-to-batch consistency.
Another key element to ensure that the strain being grown is strictly identical to the one selected years ago is to ensure the purity and consistency of the cell bank from which all new batches are produced. The pure strains, when selected are deposited in recognized culture collections (e.g.: NCIMB, Pasteur Institute..). Genetic profiling is performed to check for purity of the culture and ensure genetic identity against the deposited culture. A certificate of analysis is released for every batch of live bacteria produced by Lallemand (Figure 1).
Moreover, QC does not stop at the plant gate. The Lallemand quality team also conducts regular customer product analysis to ensure the live bacteria they purchase remain viable and active in its final application, such as feed, forage inoculants, bioremediation, and animal environment solutions.
The production process
The whole bacteria production process is schematized in Figure 1, let’s look at it in more detail.
Fermentation. It all starts from a few bacteria from a cell bank vial (1), which will be grown stepwise from a lab flask to the industrial fermenter (4).
Each bacteria strain follows its growth pattern and timeline, but a typical microbial growth curve starts with a lag phase, followed by an exponential phase (multiplication) and a plateau (stationary phase), and then a descending phase (death) (Figure 2). The aim is to halt the fermentation when the bacteria are at their most active, which is not necessarily the maximum yield.
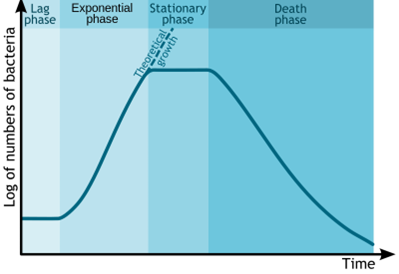
Figure 2: The bacterial growth curve represents the number of living cells in a population over time. (Michal Komorniczak/Wikimedia Commons/CC BY-SA 3.0)
Harvesting. Live bacteria are then separated from the culture medium by centrifugation: around 75% of water is removed at this stage, resulting in 20-50 times the concentration of the live bacteria (5).
Freeze drying. The preservation method used to ensure lactic bacteria stability is freeze drying – this is not used for bacilli for example. The product is first taken down to very low temperature and the remaining water is eliminated by sublimation under low pressure (sublimation is the passage from solid to gaseous phase without a liquid intermediary) (7). This method was introduced in the 1940s by Institut Rosell in Canada (today part of Lallemand Health Solutions) who developed freeze drying for lactic acid bacteria, revolutionizing bacteria preservation and their industrial applications.
Freeze drying is a very delicate step for bacteria that are sensitive to extreme temperatures. It can affect their final viability. The bacteria are mixed with an adapted cryoprotective formula. Each bacteria strain will behave differently and have different sensitivity to freeze drying. At Lallemand, constant R&D efforts are dedicated to the optimization of cryoprotectant formula and freeze-drying cycles for each specific strain that is produced.
Freeze drying is also an energy-intensive process. The company recently replaced all the freeze-driers in its UK production site (Malvern), for more efficient energy consumption.
Downstream Processing. The freeze-dried bacteria form a solid ‘cake’, containing 2-4% water. This is ground to obtain a fine, consistent powder (8).
The bacteria powder is then ready to be further blended (9), still under strict temperature and humidity control to obtain the desired formulation. It is mixed with carriers to obtain the desired bacteria concentration.
Finally, packaging (10) is another key step and is best performed on-site, under tightly controlled conditions, and using appropriate packaging material. Indeed, storage is another critical point in live bacteria survival. Live bacteria are sensitive to moisture, and atmospheric humidity should be avoided during storage. Stringent control of temperature and humidity during packaging ensures product stability and activity. Packaging material is also very important as they have varying levels of oxygen and moisture permeability. This packaging keeps out air, gasses, and moisture.
Lallemand Quality Assurance System
All Lallemand Animal Nutrition production sites and suppliers operate under the FAMI-QS or equivalent certification system, which includes:
- GMP (good manufacturing practices)
- HACCP (hazard analysis and critical control points)
- FFFD (feed fraud vulnerability and feed defense)
It ensures compliance with EU Feed Hygiene Regulation (183/2005/EC), US FDA FSMA, and most country requirements in terms of Quality and Safety Certification. These rigorous quality assurance procedures safeguard that final products are safe, of the highest quality, and compliant with customers’ requirements.
Finally, to ensure continuous improvement of its products’ safety, quality, and efficacy, Lallemand group operates a centralized complaint system. Feedback from customers is vital in this process and so all complaint information is communicated via this system to the facilities involved as well as to senior management from the business unit and the corporate Lallemand Group, from submission to complaint resolution.
To know more about the methods used for QC tests
Strain identity
Each microbial strain is unique. Strain identity is checked at various steps along the process thanks to either classical microbiology techniques (colony morphology, bioMerieux API identification system, etc.) or genetic identification techniques. DNA extraction from the biomass and polymerase chain reaction (PCR) compared to the control strain is our standard genetic identification test to confirm that the correct strain was propagated and that the bacteria is mutation-free.
Microbial purity
Microbial purity control includes the detection of the following:
- Pathogens: Enterobacteria, Salmonella, and coli. Compliance is guaranteed on the certificate of analysis issued for each batch.
- Hygiene indicators: Foreign yeast and molds and total aerobic bacteria count.
Viability: The CFU method
CFU stands for colony forming unit. It is the standard used by microbiologists to measure the number of microorganisms able to grow and multiply in a sample, using the plate count method. The sample to assess is diluted and spread onto an agar plate. After incubation, the number of microbial colonies that have formed on the plate provides a direct count of microorganisms able to multiply. Each colony is a visible aggregate of microorganisms derived from a single live bacteria cell.
The number in the original sample can be calculated according to the dilution and volume used and is usually expressed in CFU/mL or CFU/g.
Conclusion
Bacteria production involves unique and complex processes, which require substantial and continuous R&D to fine-tune the fermentation, downstream processing, as well as packaging conditions and materials and adapt them to each of the strains produced.
Strict quality control all along the process is crucial to ensure that the end product really contains the bacteria strains indicated on the label and only those and that it is in its best shape, “alive and kicking” and ready for action. Lallemand has mastered the art of fermentation during its 100+ years of experience in yeast and bacteria production across a broad range of markets, starting from baking a century ago to expanding to very specific applications in food, beverage, health, and animal nutrition. As a primary producer of microorganisms, the company has full control over the production process from the lab to packaging. This control helps ensure the quality, safety, and consistency of the bacteria products for all customers and end-users.
Discover the process inside one of our bacteria plants!
Published Apr 3, 2023 | Updated Jun 14, 2023
Related articles
Need specific information?
Talk to an expert